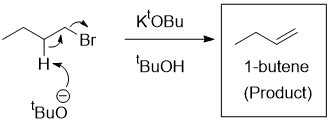
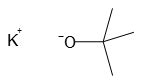
Exposing potassium t-butoxide to air can cause problems because it is a highly reactive compound that can react with moisture in the air to form t-butanol and potassium hydroxide. This reaction can release heat and cause the compound to ignite or explode. Additionally, the formation of t-butanol and potassium hydroxide can lead to the formation of hazardous fumes and vapors that can be harmful to human health. Therefore, it is important to handle potassium t-butoxide with care and store it in a dry and well-ventilated area to prevent exposure to air and moisture.
Why would exposing potassium tert-butoxide to air cause problems?
Triple-delimited paragraph:
“`Inhaling certain materials can lead to respiratory irritation, which can cause additional damage to the lungs. Corrosive bases, in particular, have been known to irritate the respiratory tract. It’s important to take precautions when working with these materials to minimize the risk of harm.“`
Is potassium tert-butoxide air sensitive?
This paragraph is a warning about the potential hazards of a chemical compound. It states that the substance is air sensitive, meaning it can react with oxygen in the air and potentially cause a fire or explosion. It also warns that the compound may form explosive peroxides, which are unstable compounds that can detonate under certain conditions. Finally, the paragraph notes that the substance is moisture sensitive, meaning it can react with water and potentially release harmful gases or heat.
Overall, this paragraph serves as a cautionary statement for anyone handling this chemical compound, emphasizing the importance of proper storage and handling procedures to minimize the risk of accidents or injuries.
What are the hazards of potassium tert-butoxide?
The paragraph provided seems to be related to a warning label for a hazardous substance. It includes hazard statements such as flammable solid and self-heating, which can cause fires, as well as precautionary statements advising to keep the substance away from heat, sparks, and flames. Additionally, it warns that the substance can cause severe skin burns and eye damage. It is important to follow these warnings to ensure safety when handling hazardous materials.
What base can be produced if potassium tert-butoxide reacts with moisture in the air?
When potassium tert-butoxide comes into contact with water, it undergoes hydrolysis, resulting in the formation of tert-butanol and potassium hydroxide. This reaction occurs easily and quickly, making it an important consideration when working with this chemical compound. It is important to handle potassium tert-butoxide with care and to take appropriate safety precautions to avoid any potential hazards.
What is the name of the product obtained when potassium reacts with air?
Potassium is a highly reactive element that readily reacts with oxygen in the air to form potassium oxide. This reaction is exothermic, meaning it releases heat. Potassium is also highly reactive with water, which can result in the production of hydrogen gas and a highly alkaline solution. Due to its reactivity, potassium is often stored in oil to prevent contact with air and moisture.
Despite its reactive nature, potassium is an essential nutrient for many living organisms, including humans. It plays a vital role in maintaining proper nerve and muscle function, regulating fluid balance in the body, and supporting healthy blood pressure levels.
What does tert-butoxide do in a reaction?
Sodium tert-butoxide has been found to be an effective mediator for coupling aryl halides with benzene derivatives. The unique aspect of this method is that it does not require the use of transition metal catalysts, which can be expensive and toxic. Instead, a catalytic 1,10-phenanthroline derivative is used to facilitate the reaction. This approach has shown promising results and offers a more sustainable and cost-effective alternative to traditional coupling methods.
What is the role of potassium tert-butoxide?
The practice of meditation has been shown to have numerous benefits for reducing stress levels in adults. Research has found that regular meditation can help to lower cortisol levels, which is the hormone associated with stress. Additionally, meditation has been shown to increase feelings of relaxation and calmness, as well as improve overall mood. By taking just a few minutes each day to meditate, individuals can experience a significant reduction in stress and anxiety levels.
So if you’re feeling overwhelmed by the demands of daily life, consider incorporating meditation into your routine to help you find peace and balance.
Is potassium tert-butoxide a strong or weak base?
It’s important to note that the tert-butoxide anion is a potent base that can react with water. As a result, it’s not possible to create a potassium tert-butoxide solution in water.
Why is potassium tert-butoxide a strong base?
Tert-butoxide, also known as (CH3)3CO−, is a highly potent base that surpasses the strength of OH− or C2H5O− ion. This is because of the presence of the +I inductive effect, which is caused by the electron-releasing methyl groups. This effect enhances the electron density of the oxygen atom, making it more nucleophilic and thus more reactive. As a result, tert-butoxide is commonly used in organic chemistry reactions, such as deprotonation and elimination reactions.
Why does tert-butoxide favor elimination?
When it comes to elimination reactions, the larger the group, the more it tends to favor this process. This is particularly true for the tert-butoxide base, which contains bulky methyl groups that donate electrons and increase the electron density of the oxygen atom through the +I effect. As a result, these groups are highly conducive to elimination reactions.
Why is tert-butoxide the strongest base?
Tert-butoxide, also known as (CH3)3CO−, is a highly potent base that surpasses the strength of OH− or C2H5O− ion. This is because of the presence of the +I inductive effect, which is caused by the electron-releasing methyl groups. This effect enhances the electron density around the oxygen atom, making it more nucleophilic and thus, more reactive. As a result, tert-butoxide is commonly used in organic chemistry reactions, such as deprotonation and elimination reactions.
Does tert-butoxide react with water?
When sodium tert-butoxide comes into contact with water, it undergoes a rapid reaction to produce sodium hydroxide. This reaction is similar to other alkaline alcoholates and occurs almost instantaneously. It is important to handle sodium tert-butoxide with care and avoid exposure to moisture to prevent this reaction from occurring prematurely. The resulting sodium hydroxide can be a useful chemical in various applications, such as in the production of soaps and detergents.
How do you remove potassium tert-butoxide?
Potassium tert-butoxide is highly reactive with moisture. To remove any impurities, it is recommended to heat the substance at around 155 ºC in a vacuum with a pressure of approximately 2 mmHg for an hour. This process will help to evaporate any tert-butanol that may be present.
What is the solvent for potassium tert-butoxide?
Meditation is a powerful tool for reducing stress levels in adults. Scientific research has shown that regular meditation practice can lead to decreased levels of cortisol, the hormone associated with stress. Additionally, meditation has been found to increase feelings of relaxation and improve overall well-being. Practicing meditation can be done in a variety of ways, such as focusing on the breath or repeating a mantra.
It is important to find a method that works best for each individual. Meditation can be easily incorporated into daily routines and can provide long-lasting benefits for managing stress.
Can a solution of potassium tert-butoxide be prepared in water?
It is not possible to prepare a solution of potassium tert-butoxide in water. This is because potassium tert-butoxide is a strong base and reacts violently with water, producing flammable hydrogen gas. Instead, it is typically prepared in anhydrous solvents such as diethyl ether or tetrahydrofuran. These solvents do not react with the base and provide a suitable environment for its use in various chemical reactions.
It is important to handle potassium tert-butoxide with care due to its reactivity and potential hazards.
What is potassium tert-butoxide as a base?
Potassium t-butoxide, also known as KOtBu, is a valuable chemical compound in organic synthesis. Its formula is [(CH3)3COK]n, and it appears as a colorless solid. This strong base has a conjugate acid with a pKa of approximately 17, making it an effective tool for various organic reactions.
Can tert-butoxide react with water?
When tert-butoxide anion comes into contact with water, it undergoes a reaction due to its strong basic properties. This reaction is significant enough to cause changes in the chemical composition of the substances involved. It is important to note that the strength of the base plays a crucial role in determining the extent of the reaction. In the case of tert-butoxide anion, its strong basic nature makes it highly reactive with water.
This reaction has been studied extensively in scientific research and is well-documented in the field of chemistry.
Is tert-butoxide a strong base or nucleophile?
Tert-butoxide, also known as tert-butoxide ion or tBuO-, is a compound that serves as the conjugate base of tert-butanol. It is commonly used as a strong base in E2 and enolate reactions, but its effectiveness as a nucleophile is limited by steric hindrance. As an illustrated glossary of organic chemistry, it is important to understand the properties and uses of tert-butoxide in various chemical reactions.
Why is potassium tert-butoxide a strong base?
Tert-butoxide, also known as (CH3)3CO−, is a highly potent base that surpasses the strength of OH− or C2H5O− ion. This is because of the presence of the +I inductive effect, which is caused by the electron-releasing methyl groups. This effect enhances the basicity of the tert-butoxide ion, making it a more effective base for chemical reactions.
Related Article
- Why Would A Guy Ask For A Picture Of You?
- Why Would A Female Deer Be Alone In The Winter?
- Why Would A City Want To Host The Final Four?
- Why Would A Business Owner Likely Use A Java Applet?
- Why Wont My Blades Engage On My John Deere Mower?
- Why Won’T Shein Accept My American Express Gift Card?
- Why Won’T My Volume Go All The Way Down?
- Why Won’T My Ps4 Connect To My Roku Tv?
- Why Won’T My Led Lights Connect To Lotus Lantern?
- Why Won’T My Dog Go In The Grass Anymore?