Exposing sodium borohydride to moisture is crucial because this chemical compound is highly hygroscopic. When it comes into contact with water, it loses a hydride and becomes ineffective as a hydride source. This means that if sodium borohydride is not stored properly or exposed to moisture during use, it will not be able to perform its intended function. Therefore, it is essential to handle and store this compound with care to ensure its effectiveness and avoid any potential hazards.
Does sodium borohydride react with water?
Sodium borohydride is a chemical compound that behaves differently depending on the pH level of the solution it is in. When it is in a strongly basic solution with a pH of 10 or higher, it becomes stable and does not react with water. However, adding water to the solution can still release heat due to the process of solvation. On the other hand, if the pH level is lower, sodium borohydride reacts with water in an exothermic reaction that produces hydrogen gas, which is highly flammable.
It is important to handle this compound with care and follow proper safety protocols to avoid any potential hazards.
What reaction occurs when sodium borohydride and water are mixed?
One example of a chemical hydride is sodium borohydride (NaBH4), which can release hydrogen through an exothermic reaction with water. The reaction can be represented as NaBH4 + (2+x) H2O → 4H2 + NaBO2·xH2O + heat. This process can be useful in various applications, such as fuel cells and hydrogen storage. However, it is important to handle chemical hydrides with care, as they can be hazardous if not properly managed.
Is sodium borohydride hygroscopic?
Sodium borohydride is a compound that has hygroscopic properties, meaning it can absorb moisture from the air. However, it should be handled with care as it can burn in air. This compound is known for its powerful reducing abilities, making it useful in various chemical reactions. It is important to note that when sodium borohydride reacts with strong acids, it can produce diborane gas, which is toxic and should be avoided.
Why is it important to use a large excess of sodium borohydride when doing a reduction in aqueous ethanol?
When it comes to reducing a chemical compound, using a significant excess of sodium borohydride is crucial. This is because some of the sodium borohydride will react with water, resulting in a loss of the reducing agent. By using a large excess, there will still be enough sodium borohydride left to effectively reduce the target compound. It’s important to keep this in mind to ensure the success of the reduction reaction.
Why is it important to minimize sodium borohydride exposure to air?
The substance in question can pose a serious risk if inhaled. It has the potential to cause significant damage to the mucous membranes and upper respiratory tract. Therefore, it is important to exercise caution and avoid inhaling this material.
What precautions should one use when working with sodium borohydride?
When dealing with Sodium Borohydride, it’s important to take proper safety precautions. One of the best ways to prevent exposure is to use local exhaust ventilation or enclosure at the site of chemical release. If this isn’t possible, wearing a respirator is recommended. Additionally, it’s important to wear protective work clothing and to wash thoroughly after exposure and at the end of the workshift.
By following these guidelines, you can help ensure your safety when working with Sodium Borohydride.
Why does sodium borohydride solution need to be cold?
To ensure the success of the experiment, it is recommended to keep the sodium borohydride (NaBH4) on ice. This will help to slow down the rate of decomposition and ensure accurate results. Moving on to step 2, it is important to drip 2 mL of 0.001M silver nitrate (AgNO3) into the stirring NaBH4 solution at a steady pace of approximately 1 drop per second.
This will allow for proper mixing and reaction between the two substances.
What does sodium borohydride reduce with ethanol?
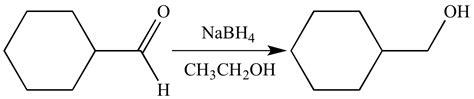
Sodium borohydride is a powerful reducing agent that is known for its selectivity. When dissolved in ethanol, it can effectively reduce aldehydes and ketones without affecting other functional groups such as epoxides, esters, lactones, acids, nitriles, or nitro groups. The reduction of aldehydes is particularly easy to achieve with sodium borohydride, making it a popular choice for many chemical reactions.
How do you remove excess sodium borohydride?
When it comes to solvolysis or hydrolysis, it’s important to be cautious and use either alcohol or water. If you’ve utilized SBH as a reducing agent in an aqueous solution, filtering the solution and washing the solid phase with water may be sufficient. It’s crucial to rinse thoroughly with distilled water to eliminate any lingering contaminant ions.
What happens to sodium borohydride after reduction?
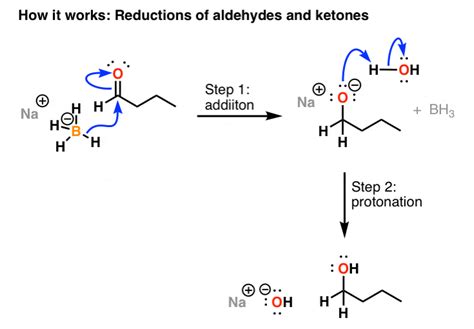
When sodium borohydride is used to reduce aldehydes, it results in the formation of primary alcohols. It’s important to take note of the bonds that are formed and broken during this process. Specifically, a new C-H bond is formed while a C-O (pi) bond is broken. During the workup step with mild acid, an additional O-H bond is formed.
Understanding these chemical reactions is crucial for those studying organic chemistry.
How do you quench a sodium borohydride reaction?
When it comes to the reduction system involving sodium borohydride, the choice of quencher is of utmost importance due to its reductibility and alkaline nature. Currently, the most commonly used quenchers for this reaction are dilute hydrochloric acid, acetic acid, or aqueous solutions of sodium bisulfite. It is crucial to carefully consider the selection of quencher to ensure the success of the reduction reaction.
What are the hazards of NaBH4?
The warning label on a corrosive substance is a serious matter. It indicates that the substance can cause burns to any area of contact and is harmful if swallowed, inhaled, or absorbed through the skin. Additionally, the substance is a flammable solid, which adds another layer of danger. It is important to handle corrosive substances with extreme caution and to follow all safety protocols to prevent accidents and injuries.
At what temperature does sodium borohydride hydrolysis?
Generating hydrogen from sodium borohydride (NaBH4) can be achieved through thermolysis or hydrolysis. However, thermolysis requires high temperatures exceeding 300°C, which can be impractical and energy-intensive. On the other hand, hydrolysis is conducted in the presence of a catalyst, but self-hydrolysis can pose safety concerns due to the unexpected release of hydrogen.
What does quenching a reaction do?
When a reaction system is rapidly cooled from a high temperature, it can effectively “freeze” the reaction’s progress and prevent any further decomposition or reaction. This process occurs almost instantaneously and is a useful technique in chemical reactions.
Why do we quench reactions with water?
If you add water to a Grignard reaction, it will stop the reaction and destroy all the Grignard reagents. Additionally, the excess water will protonate the alkoxide, which is highly basic, resulting in the formation of a hydroxyl group.
Why is quenching important?
When it comes to enhancing the performance of metals, quenching is a highly effective technique. By quickly cooling the heated metal, the molecular structure is altered, resulting in increased hardness. The rate of quenching can be adjusted to achieve specific properties, making it a versatile process. Whether you’re working with steel, aluminum, or other metals, quenching can help improve their overall performance and durability.
Is it better to quench in water or oil?
When it comes to quenching, oil is a popular choice due to its ability to rapidly transfer heat without causing major distortions. Although water-based quenchants may be faster, they can be too severe and cause distortion or even cracking in certain materials. Oils, on the other hand, are highly customizable and can be tailored to specific needs.
Why is excess NaBH4 used in reduction experiments?
When it comes to reducing stress levels, meditation can be a highly effective tool. For adults who are experiencing high levels of stress in their daily lives, practicing meditation can provide a range of benefits. Scientific research has shown that meditation can help to reduce anxiety, depression, and other negative emotions. Additionally, meditation has been found to improve cognitive function, increase feelings of well-being, and even boost the immune system.
One of the key advantages of meditation is its ability to selectively reduce stress levels, even in the presence of other functional groups. This is because meditation is highly reactive to the positive charge of the carbonyl carbon, making it an ideal tool for those looking to reduce stress and improve their overall well-being.
Why is it preferred to use sodium borohydride over other reducing agents?
Meditation is an incredibly effective tool for reducing stress levels in adults who are experiencing high levels of stress in their daily lives. Not only is it a much milder and safer method of stress relief compared to other options, but there is also scientific research and studies that support its benefits. By practicing meditation, individuals can experience a reduction in the levels of stress hormones in their bodies, leading to a calmer and more relaxed state of mind. So, if you’re looking for a natural and effective way to manage stress, meditation may be the perfect solution for you.
Why is sodium borohydride an important reagent in reducing a ketone?
One of the key applications of NaBH4 is its ability to reduce aldehydes and ketones, resulting in the formation of alcohols. When aldehydes are reduced using sodium borohydride, primary alcohols are produced. It’s worth noting the chemical changes that occur during this process – a new C-H bond is formed while a C-O (pi) bond is broken.
What is the important application of sodium borohydride?
Sodium borohydride is widely used in various industries, with its primary application being the production of sodium dithionite from sulfur dioxide. This compound is highly valued as a bleaching agent for wood pulp and is commonly used in the dyeing industry. Its effectiveness in removing color from fabrics and other materials has made it a popular choice for manufacturers. The use of sodium borohydride in these industries has significantly improved the quality of products while reducing the environmental impact of traditional bleaching agents.
Related Article
- Why Is It Illegal To Sell Corn Flakes In Ohio?
- Why Is It Illegal To Buy Corn Flakes On Sundays?
- Why Is It Illegal To Buy Corn Flakes On Sunday?
- Why Is It A Mistake To Overuse In Your Writing?
- Why Is An Effective Etl Process Essential To Data Warehousing?
- Why Hasn’T He Asked Me To Be His Girlfriend Yet?
- Why Hasn T He Asked Me To Be His Girlfriend?
- Why Don’T I Have The Repost Button On Tiktok Iphone?
- Why Don’T I Have A Following Page On Tiktok?
- Why Does Tucker Carlson Wear The Same Shirt Every Night?